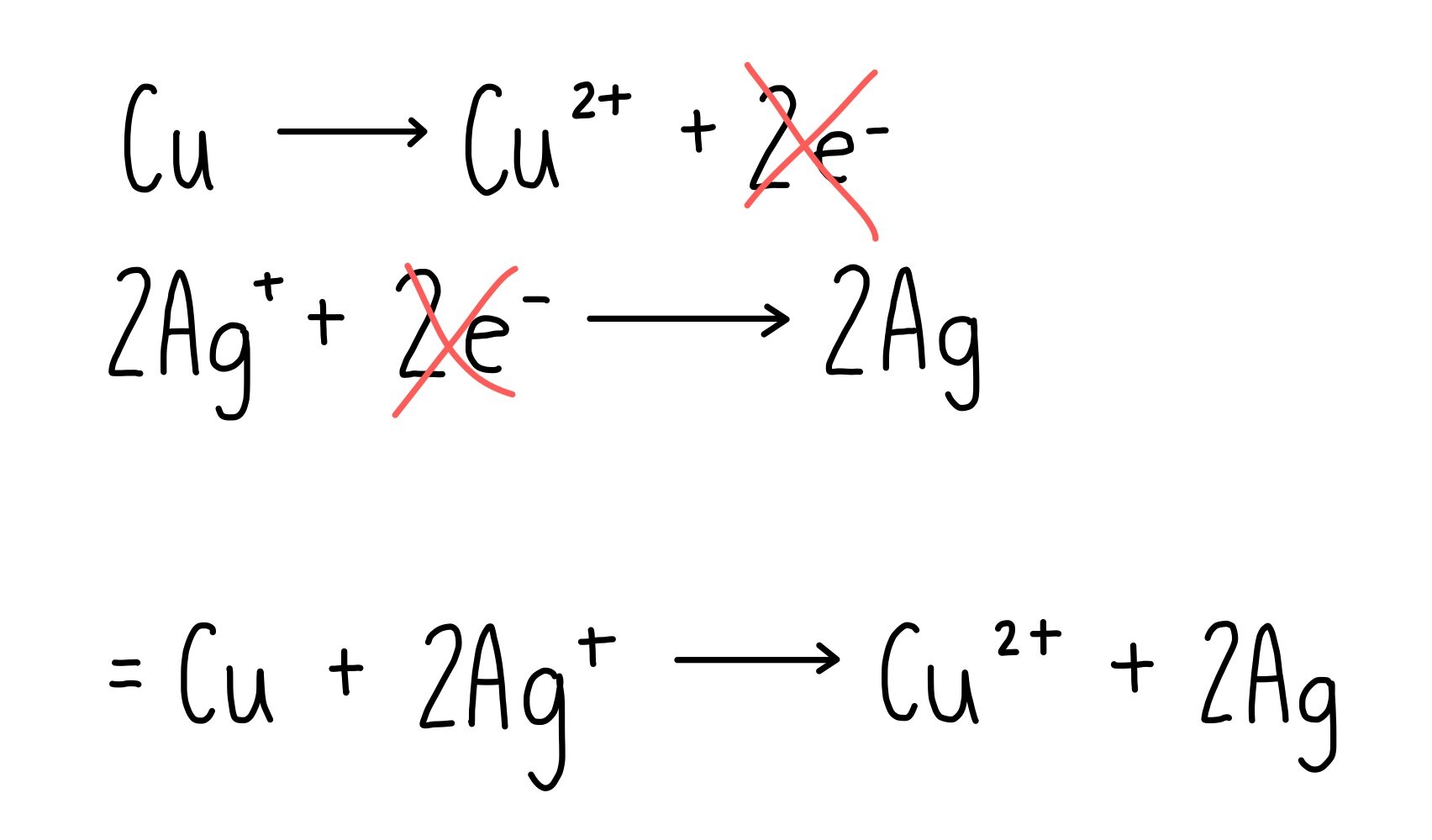Copper Equation Oxygen . The copper oxide can then react with the hydrogen. For example, copper and oxygen react. Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. heated copper metal reacts with oxygen to form the black copper oxide. explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. balanced symbol equations show what happens to the different atoms in reactions. Includes kit list and safety instructions. two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation.
from www.thesciencehive.co.uk
Includes kit list and safety instructions. balanced symbol equations show what happens to the different atoms in reactions. two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. heated copper metal reacts with oxygen to form the black copper oxide. For example, copper and oxygen react. The copper oxide can then react with the hydrogen. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation.
Redox and Electrode Potentials — the science hive
Copper Equation Oxygen two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. balanced symbol equations show what happens to the different atoms in reactions. Includes kit list and safety instructions. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation. heated copper metal reacts with oxygen to form the black copper oxide. two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. For example, copper and oxygen react. The copper oxide can then react with the hydrogen.
From www.numerade.com
SOLVEDOxidation of bis(bipyridine)copper(I) ion by molecular oxygen is described by the Copper Equation Oxygen copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. heated copper metal reacts with oxygen to form the black copper oxide. two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. For example, copper and oxygen react.. Copper Equation Oxygen.
From www.numerade.com
SOLVED 12.0 g of copper is concerted to copper (II) oxide by heating in oxygen. Write a Copper Equation Oxygen copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. For example, copper and oxygen react. to balance cu + o2 = cuo you'll need to be sure to count all. Copper Equation Oxygen.
From www.numerade.com
SOLVEDCopper(I) sulfide reacts with O2 upon heating to give copper metal and sulfur dioxide. (a Copper Equation Oxygen For example, copper and oxygen react. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation. explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. Includes kit list and safety instructions. . Copper Equation Oxygen.
From www.sarthaks.com
When hydrogen gas is passed over heated copper (II) oxide, copper and steam are formed. Write Copper Equation Oxygen Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. For example, copper and oxygen react. heated copper metal reacts with oxygen to form the black copper oxide. to balance. Copper Equation Oxygen.
From www.youtube.com
Copper Vs Oxygen Reaction YouTube Copper Equation Oxygen The copper oxide can then react with the hydrogen. heated copper metal reacts with oxygen to form the black copper oxide. explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. balanced symbol equations show what happens to the different atoms in reactions. For example, copper. Copper Equation Oxygen.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Copper Equation Oxygen two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. balanced symbol equations show what happens to the different atoms in reactions. Use this class practical with your. Copper Equation Oxygen.
From www.numerade.com
SOLVEDWrite a balanced chemical equation for each chemical reaction. (a) Solid copper reacts Copper Equation Oxygen balanced symbol equations show what happens to the different atoms in reactions. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. The copper oxide can then react with the hydrogen. heated copper metal reacts with oxygen to form the black copper oxide. Use this class practical with your students. Copper Equation Oxygen.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free download ID6645971 Copper Equation Oxygen copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. balanced symbol equations show what happens to the different atoms in reactions. to balance cu + o2 =. Copper Equation Oxygen.
From stewart-switch.com
Copper Oxygen Phase Diagram Copper Equation Oxygen two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. Includes kit list and safety instructions. For example, copper and oxygen react. to balance cu +. Copper Equation Oxygen.
From www.youtube.com
How to Write the Formula for Copper (I) oxide YouTube Copper Equation Oxygen For example, copper and oxygen react. heated copper metal reacts with oxygen to form the black copper oxide. balanced symbol equations show what happens to the different atoms in reactions. two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. to balance cu +. Copper Equation Oxygen.
From www.youtube.com
Oxidation of Copper to Copper Oxide Class X Science Chapter1Chemical reactions & equations Copper Equation Oxygen For example, copper and oxygen react. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. Use this class practical with your students to deduce the formula. Copper Equation Oxygen.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation ID5482147 Copper Equation Oxygen heated copper metal reacts with oxygen to form the black copper oxide. two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. copper (i) chemistry. Copper Equation Oxygen.
From www.numerade.com
Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous sulfur trioxide and solid Copper Equation Oxygen balanced symbol equations show what happens to the different atoms in reactions. For example, copper and oxygen react. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions in solution. The copper oxide can then react with the hydrogen. two atoms of copper react with one molecule of oxygen (which is made. Copper Equation Oxygen.
From kunduz.com
[ANSWERED] F. copper (Cu) and oxygen (O₂) (4 pt.) balanced equation Kunduz Copper Equation Oxygen to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation. For example, copper and oxygen react. heated copper metal reacts with oxygen to form the black copper oxide. copper (i) chemistry is limited by a reaction which occurs involving simple copper (i) ions. Copper Equation Oxygen.
From www.transtutors.com
(Solved) The Unit Cell Of A Binary Compound Of Copper And Oxygen Is Shown... (1 Answer Copper Equation Oxygen two atoms of copper react with one molecule of oxygen (which is made of two atoms) to form two molecules of copper. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation. balanced symbol equations show what happens to the different atoms in. Copper Equation Oxygen.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation, free download ID4272546 Copper Equation Oxygen Use this class practical with your students to deduce the formula of copper (ii) oxide from its reduction by methane. balanced symbol equations show what happens to the different atoms in reactions. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation. two. Copper Equation Oxygen.
From www.vrogue.co
Schematic Phase Diagrams Of Copper Oxygen Left Two Pa vrogue.co Copper Equation Oxygen heated copper metal reacts with oxygen to form the black copper oxide. Includes kit list and safety instructions. to balance cu + o2 = cuo you'll need to be sure to count all of atoms on each side of the chemical equation. For example, copper and oxygen react. two atoms of copper react with one molecule of. Copper Equation Oxygen.
From brainly.in
balanced equation for copper powder is heated strongly in air and copper oxide is formed also Copper Equation Oxygen For example, copper and oxygen react. balanced symbol equations show what happens to the different atoms in reactions. The copper oxide can then react with the hydrogen. explore the reaction of copper with oxygen, producing copper oxide, when a copper envelope is heated in air in this practical. two atoms of copper react with one molecule of. Copper Equation Oxygen.